In the last post we have
discussed the reasons behind the non-ideal behaviour of real gases. As we
behave ideally in certain conditions, real gases also do. What are those
conditions in which real gases behave like an Ideal gas?
As you know intermolecular forces are responsible for the non-ideal behaviour of real gases. So think about those situations in which these forces have no significance. Inter molecular forces are effective within a small range, if we increase the distance between two molecules, Inter molecular forces will diminish.
In Boyle’s Law you have learnt that at lower pressure the volume of gas increases, which in turn increases the distance between molecules and in the larger space, volume of molecules can be neglected. So by decreasing pressure we can beat attractive force by increasing the distance.
But still molecules are not
completely free from attractive force; there are chances that they can be
affected by the attractive force if any of them passes a nearby molecule. To
cancel this possibility of getting caught by attractive force of nearby
molecule, we have to increase the speed (or kinetic energy) of molecules and to
increase their kinetic energy we have to increase temperature. At higher
temperature molecules travel with higher speed and wouldn't get caught by other
molecule.
That means at lower pressure and
higher temperature real gases behave like an ideal gas. The temperature at
which a real gas obeys Boyle’s law is called the Boyle’s temperature or Boyle’s
point. Boyle’s point of gas depends on it’s nature.
Now we know how we can modify
conditions to force real gases to behave ideally. Can we measure their
deviation from ideal behaviour?
pV = nRT
pV/ nRT = 1
For an ideal gas the ratio of pV/
nRT is equal to 1. But in case of real gases this ratio deviates from unity.
This ratio is defined as ‘Compressibility Factor’ and denoted as Z.
pV/ nRT = Z
When a graph is plotted between Z
and pressure, we get a straight line for ideal gas. And for real gases we get
different curves showing positive or negative deviation from straight line.
If we derive another equation
from it, you will be able to understand the Compressibility Factor better.
pV/ nRT = Z
if we write V as Vreal
p Vreal / nRT = Z
From ideal gas equation we know:
pVideal = nRT
Videal = nRT/p
Now place the value of nRT/p in
the above equation:
Vreal / Videal
= Z
In a graph of Z vs Pressure for ideal gas, we get a straight line at Z=1 which is parallel to the x axis. It doesn’t mean that with increase in pressure no changes occur to ideal gas, changes occur but these changes occur in such a manner that the ratio of pV/nRT remains unity.
While in case of real gases these changes occur in undisciplined way and the ratio of pV/nRT deviates from unity. Most of the real gases show a three staged curve where they have Z ≈1 at lower pressure, Z < 1 at high pressure and Z > 1 at a still higher pressure.
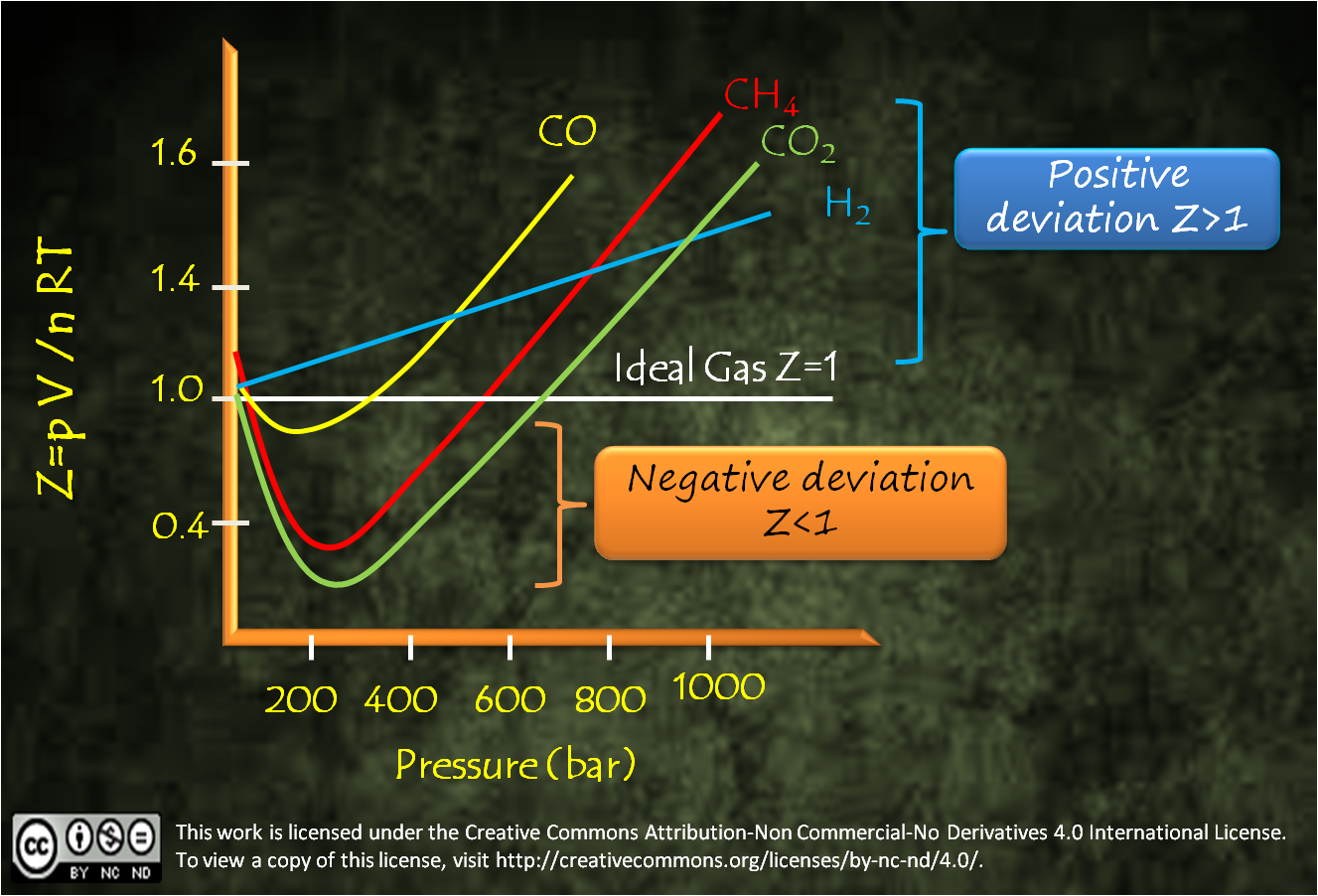
Stage I:
At lower pressure
where Z ≈ 1 all gases show
ideal behaviour. As pressure increases most of the real gases show
negative deviation where Z <
1, which means Vreal is
less than Videal which
signifies that the gas gets compressed more than the ideal gas at increased
pressure. Here first time real gases start disobeying the Boyle’s law (p ∝ V-1).
Stage II: As the pressure further increases, all
real gases touch the straight line for an instance when they have Z= 1 and where they behave ideally
since Vreal is
equal to Videal.
Stage III: As the pressure reaches to still higher range,
all real gases again deviate from ideal behaviour and show positive deviation
where Z > 1. At this
stage Vreal is
more than Videal that
means the gases no more follow the trend of Boyle’s law of decrease in volume
on increasing pressure. At this stage real gases again start disobeying the
Boyle’s law (p ∝ V-1) and it becomes impossible to compress
them.
Now you understand that why real
gases obey Ideal gas equation at lower pressure and higher temperature. Does
this information benefit to us? In the next post we will see how we can use it
in practical life?
Related Posts-

This work is licensed under the Creative Commons Attribution-Non
Commercial-No Derivatives 4.0 International License. To view a copy of this
license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Comments
Post a Comment